Methanol-Acetone Extractive Distillation Plant
- Home
- Petrochemical Plant
- Methanol-Acetone Extractive Distillation Plant
Fundamental
 Acetone and methanol are also commonly used organic solvents in the pharmaceutical industry, and the pharmaceutical process often involves the separation, recovery and reuse of the mixed solution of acetone and methanol. Since methanol (boiling point 64.7 ℃) and acetone (boiling point 56.5 ℃) are similar in boiling point, it is easy to form an azeotrope, so it is difficult to separate it by general distillation. Extractive distillation is widely used as a common method for separating azeotropes, so the choice of extraction efficiency has become the top priority of extractive distillation.
Acetone and methanol are also commonly used organic solvents in the pharmaceutical industry, and the pharmaceutical process often involves the separation, recovery and reuse of the mixed solution of acetone and methanol. Since methanol (boiling point 64.7 ℃) and acetone (boiling point 56.5 ℃) are similar in boiling point, it is easy to form an azeotrope, so it is difficult to separate it by general distillation. Extractive distillation is widely used as a common method for separating azeotropes, so the choice of extraction efficiency has become the top priority of extractive distillation.
Rectification is the process of performing partial gasification and partial condensation multiple times, which can make the mixed liquid almost completely separated. After performing partial vaporization or partial condensation multiple times, more pure volatile components can be finally obtained in the vapor phase In the liquid phase, relatively pure, less volatile components are obtained.
Rectification is generally divided into continuous rectification and batch rectification. In the continuous rectification process, the feed liquid is continuously added to the rectification tower from a suitable position in the middle of the tower. A condenser is provided at the top of the tower to condense the top steam into a part of the liquid condensate and return to the top of the tower, called reflux liquid The top product, namely the distillate, is continuously discharged. In the upper half of the tower above the charging position, countercurrent contact and mass transfer are performed between the rising steam and the reflux liquid. The bottom of the tower is equipped with a reboiler (distillation kettle) to heat the liquid to generate steam. The steam rises along the tower, contacts the falling liquid countercurrently and conducts mass transfer. The bottom of the tower continuously discharges part of the liquid as the bottom product. Above the feed position of the tower, the heavy components contained in the rising steam are transferred to the liquid phase, while the light components in the reflux liquid are transferred to the gas phase.
VMETS Extractive distillation plant of methanol and acetone mixture
Since the boiling points of methanol and acetone are close, the azeotrope formed is separated, and an extractant (such as an aqueous solution) must be added to reuse condensed water with a certain temperature. On the one hand, it saves costs and controls energy consumption, on the other hand, it can avoid the formation of scale ), Using the difference in relative volatility of the azeotrope formed by mixing methanol or acetone with the extractant, through the material transfer of the extraction tower, the mixture formed by acetone and extractant is first separated from the top of the extraction tower to the acetone rectification tower, With the continuous purification of acetone, the content of methanol in the mixture of acetone and methanol is getting higher and higher. It is necessary to increase the amount of extractant, increase the separation of methanol and acetone, and then obtain methanol with higher purity through the methanol rectification tower.
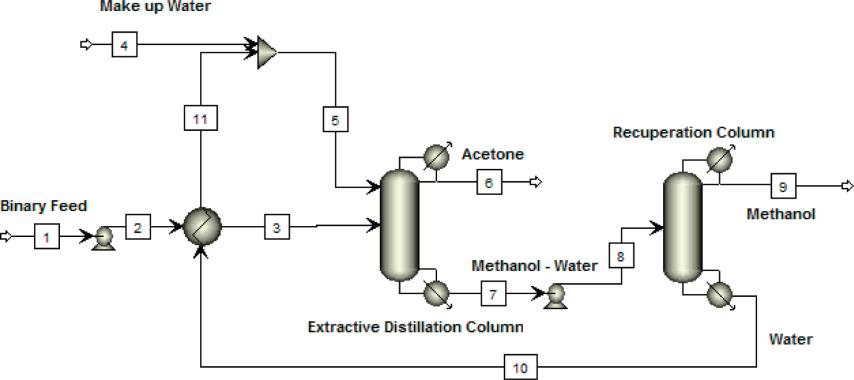
Process flow-sheet for VMETS extractive distillation of acetone-methanol using water as an entrainer.
