Hydrodynamic Cavitation Desulfurization Plant
- Home
- Desulfurization Plant
- Hydrodynamic Cavitation Desulfurization Plant
Hydrodynamic cavitation
Hydrodynamic cavitation describes the process of vaporisation, bubble generation and bubble implosion which occurs in a flowing liquid as a result of a decrease and subsequent increase in local pressure. Cavitation will only occur if the local pressure declines to some point below the saturated vapor pressure of the liquid and subsequent recovery above the vapor pressure. If the recovery pressure is not above the vapor pressure then flashing is said to have occurred. In pipe systems, cavitation typically occurs either as the result of an increase in the kinetic energy (through an area constriction) or an increase in the pipe elevation.
Hydrodynamic cavitation can be produced by passing a liquid through a constricted channel at a specific flow velocity or by mechanical rotation of an object through a liquid. In the case of the constricted channel and based on the specific (or unique) geometry of the system, the combination of pressure and kinetic energy can create the hydrodynamic cavitation cavern downstream of the local constriction generating high energy cavitation bubbles.
The process of bubble generation, and the subsequent growth and collapse of the cavitation bubbles, results in very high energy densities and in very high local temperatures and local pressures at the surface of the bubbles for a very short time. The overall liquid medium environment, therefore, remains at ambient conditions. When uncontrolled, cavitation is damaging; by controlling the flow of the cavitation, however, the power can be harnessed and non-destructive. Controlled cavitation can be used to enhance chemical reactions or propagate certain unexpected reactions because free radicals are generated in the process due to disassociation of vapors trapped in the cavitating bubbles.
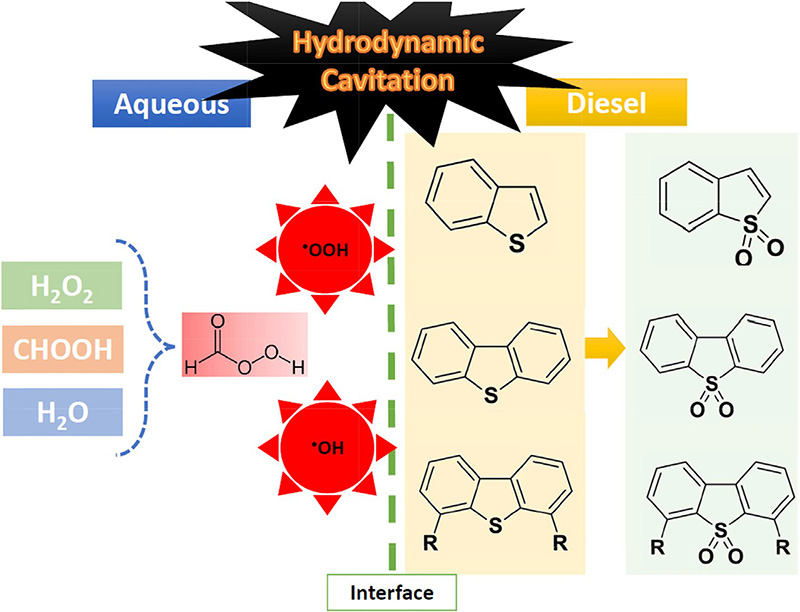
Schematic pathway of diesel desulfurization via HCAOD
Introduction of Hydrodynamic Cavitation Assisted Oxidative Desulfurization (HCAOD) process
Hydrodynamic Cavitation (HC) has been extensively studied by researches as a promising intensification technique for degradation of organic contaminants in wastewater . Mechanistic feature of the phenomenon is relatively well known dealing with water. However, a considerable challenge in this area of the study, is the generation of HC and its subsequent effects in the nonaqueous mediums. Some researchers have demonstrated utilization of HC in biodiesel production. Since the reaction of the two immiscible phases (i.e., oil/fat and alcohol) is controlled by the mass transfer limitations and physical effects of HC such as micro mixing, intense turbulence and streaming can be effective in promoting kinetic of the reaction leading to increased efficiency and decrease reaction time. Recently, a novel approach is utilized for deep desulfurization process using hydrodynamic cavitation. The authors have investigated the ability of oxidizing sulfur compound through hydrocarbon (n-octane, toluene, n-octanol and commercial diesel)-water mixture via HC and utilized vortex diode as the cavitating device. According to their conclusions, hydrodynamic cavitation processes have the potential of bringing up a green approach for desulfurization of fuels without the use of catalyst or external chemicals or reagents and seems to be economically sustainable.
Sulphur containing organic phase is mixed with water under ambient conditions and passed through a vortex diode28. Vapor cavities are formed in the diode and are transported to the downstream region where these cavities collapse. The cavity collapse generates localized very high pressure and temperature29 as well as hydroxyl radicals. Interaction of hydroxyl radicals under these locally extreme conditions result in removal of sulphur from the organic phase without any catalyst under apparently ambient conditions of bulk. A schematic of vortex diode functioning, possible steps in desulphurization using cavitation process and photograph of the experimental set-up for carrying out the experiments of the developed process are shown in Fig. 1.
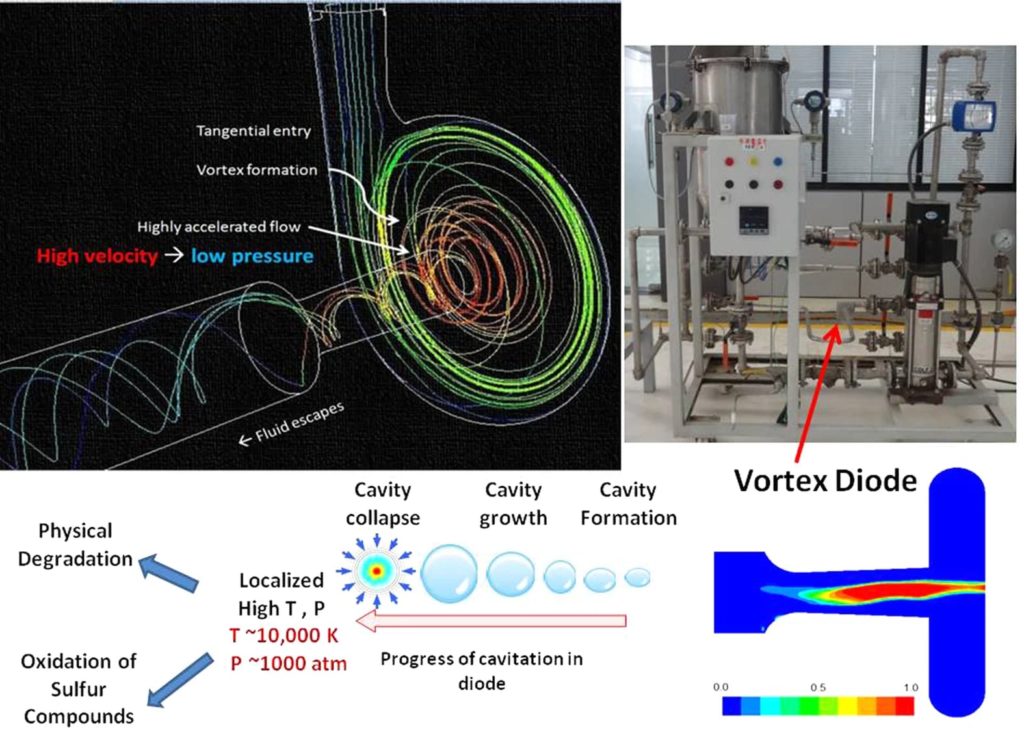
Figure 1
